
SL Paper 1
Which descriptions are correct for both a Brønsted–Lowry acid and a Lewis acid?
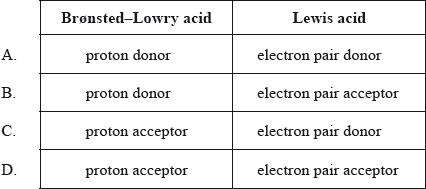
Markscheme
B
Examiners report
Which definition of a base is correct?
A. A Lewis base accepts a proton.
B. A Brønsted-Lowry base accepts an electron pair.
C. A Brønsted-Lowry base donates an electron pair.
D. A Lewis base donates an electron pair.
Markscheme
D
Examiners report
Which substance can act as a Lewis acid but not as a Brønsted–Lowry acid?
A. HCl
B. CH3COOH
C. BF3
D. CF3COOH
Markscheme
C
Examiners report
Which are definitions of an acid according to the Brønsted-Lowry and Lewis theories?
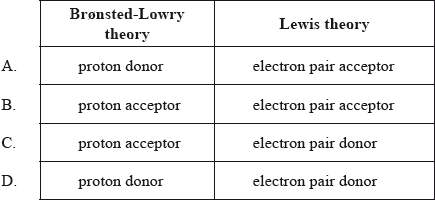
Markscheme
A
Examiners report
Which species cannot function as a Lewis acid?
A. \({\text{B}}{{\text{F}}_{\text{3}}}\)
B. \({\text{AlC}}{{\text{l}}_{\text{3}}}\)
C. \({\text{CC}}{{\text{l}}_{\text{4}}}\)
D. \({{\text{H}}^ + }\)
Markscheme
C
Examiners report
Whilst 42% correctly identified \({\text{CC}}{{\text{l}}_{\text{4}}}\) the most popular wrong answer (38%) was the \({{\text{H}}^ + }\) ion.
Which statement explains why ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is classified as a Lewis base?
A. It can accept a proton.
B. It can accept a lone pair of electrons.
C. It can donate a lone pair of electrons.
D. It can donate a proton.
Markscheme
C